| Corporate address | 265 State Route 34, Bldg 2 Colts Neck, NJ 07721 |
| Mailing address | P.O. Box 162 Middletown, NJ 07748 |
Case Study
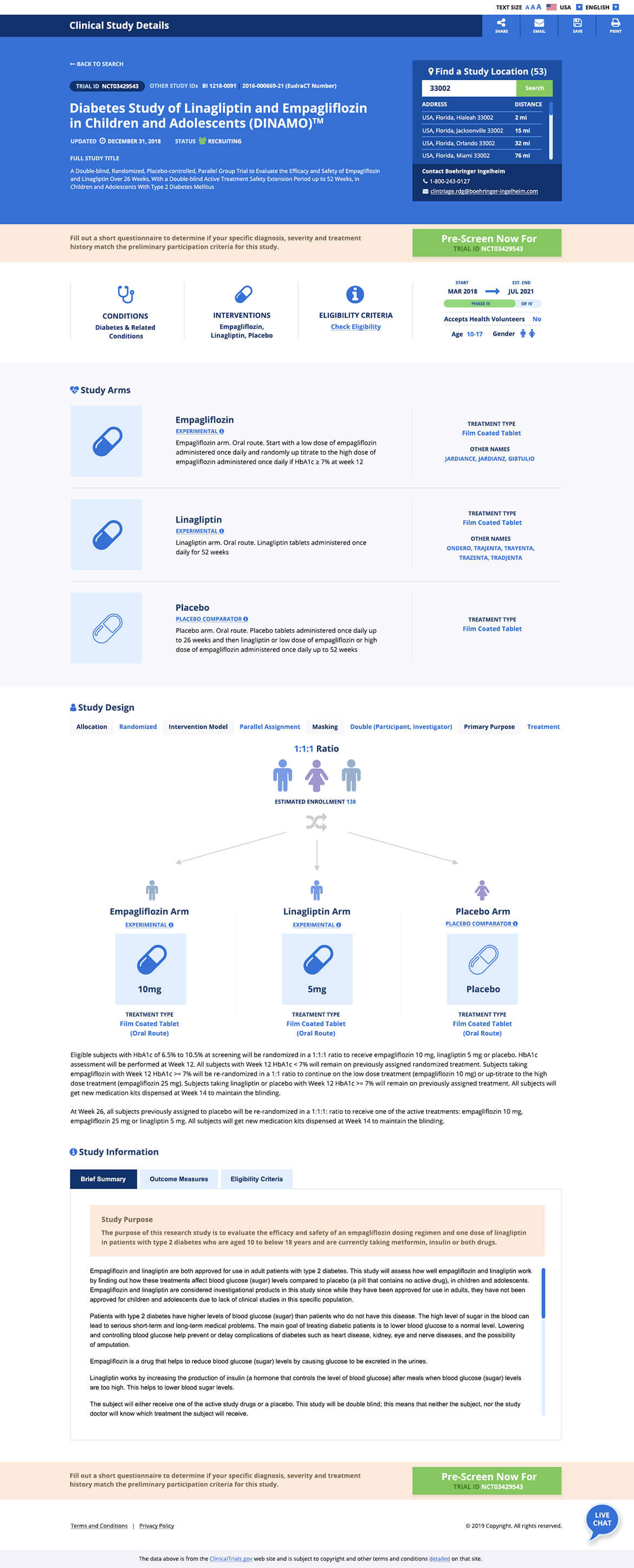
Clinical trial websites are often the first stop for an individual to visit when they are considering participation in a research study. In fact, according to the Center for Information and Study on Clinical Research Participation (CISCRP), the top way that individuals find out about clinical trials is online. And the number one way that organizations can recruit these people and turn them into clinical trial participants, is through a website.
It’s so much more than aesthetics. A good design is all about communicating critical information needed for patients, caregivers and physicians. Separately, the ability to understand key salient points from previously conducted studies to understand how they would affect them and their future treatment.
What We Did
CMDS/Esperienza was approached by an international pharmaceutical company in late 2018 to help guide them along in their process of creating a new patient-centric web platform that helped support patient needs and interests in finding information on clinical trials for their conditions. CMDS was sought as a partner for this exercise because of their fundamental understanding of the five foundational design considerations for clinical trial websites.
The initial exercise included the CMDS creative team flying to the company’s corporate headquarters in Germany to meet with the strategic stakeholders of the clinical trial program. CMDS was instrumental in leading several day-long sessions to truly explore the pain points and challenges that various audiences face when becoming involved in the clinical trial process. The collaborative exercise helped outline the overall goals of the new site for the company, including:
As an output of the creative brainstorming session, there was an agreed upon focus to consider three key personas: Patient, Caregiver, and Medical Professional. CMDS was able to explore first-hand data as it related to what questions these key personas typically had when exploring clinical trials.
By digging into the pain points of these personas and humanizing their challenges, concerns and opportunities, CMDS began to shape the understanding and breakdown of what was important to each. Content was assessed for how it should be prioritized based on each persona’s need and attention span. Cultural differences were considered in how layouts, color, and imagery were to be used.
“I’ve been diagnosed with this health condition, please send me all relevant information needed to participate in your study”
“I would like the name of the physician at this location to approach regarding recruitment into this trial to treat my health condition”
“I believe my daughter meets the eligibility requirements for this trial. She was diagnosed with this health condition 3 years ago and has pursued the required courses of treatment thus far with no success”
“My mother is currently on these drugs and it seems not to be working. Do you think this trial can help her?”
“My patient matches the eligibility criteria for this trial. Please assist with getting them more information in participating in the trial.”
“I would like to explore the possibility of expanding the study at our site.”

CMDS was then able to initiate the UX Planning process. We considered the current clinicaltrials.gov website as a baseline for what the clinical trials exploratory process exists as today. That site required much digging to find content related to your specific health condition and there is no immediate tie to what health condition a trial is treating and no understanding of where the trial is taking place. We looked to prioritize certain items based on what would be important to the individuals visiting the site, including:

Content was studied as individual elements and those pieces were reordered based on the level of interest/importance to users. Desktop vs. mobile usage was considered in both the design and structure.
Other items were considered in the usability planning process including:
Finally the design planning stage began where visual considerations were made based on the key personas. We utilized the findings to place elements on a page graphically. Colors were chosen based on the identified personas. Blue was utilized for our example piece as it is viewed as reliable and responsible. The color reduces stress, creating a sense of calmness, relaxation and order. The shapes that were utilized had strategic meanings. Squares were utilized for our example piece as they are viewed as reliable, stable, and suggest order. CMDS studied heatmaps on other clinical trial websites to determine where content blocks must be positioned based on natural user behavior.
CMDS was proud to showcase the final design to the client, and they were impressed with the final outcome for their world-class clinical trial platform.
Discover how a strategic website design can help to accelerate clinical trial participation with patient enrollment and sponsorship opportunities. Keep clinical trial patients and sponsors engaged every step of the way - from first impressions through enrollment.
At CMDS, we have extensive experience working with pharma and healthcare brands nationwide. From website copywriting and SEO to website design & development, our experts know how to navigate this challenging space. Give us a call at 732.706.5555 or fill out this online form to speak with a website strategist today.